





- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - -

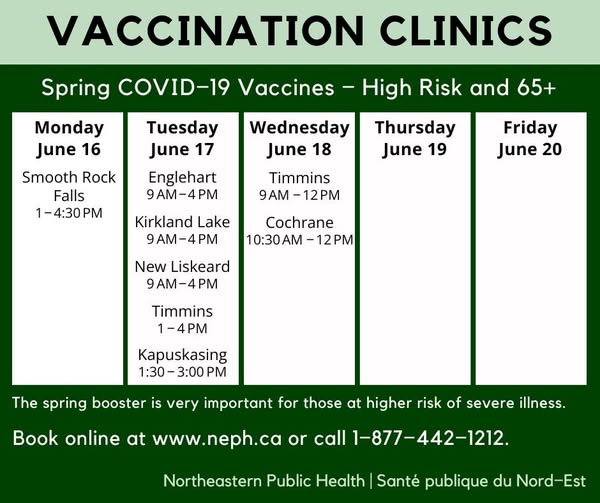
COVID-19 Vaccination Clinics in Timiskaming
COVID-19 Spring Program:
Week of June 16th:
The following groups are recommended to get a spring dose:
- Adults 65 years of age and older:
- NACI recommends that those 80 years and older should receive an additional dose of vaccine, while those 65 to 79 yers of age may receive an additional dose of vaccine.
- Adult residents of long-term care homes and other congregate living settings for seniors
- Individuals 6 months of age and older who are moderately to severely immunocompromised (due to underlying condition or treatment)
- Individuals 55 years and older who identify as First Nations, Inuit, or Metis and their non-Indigenous household members who are 55 years and older.
NOTE:
- Please make an appointment for every family member wanting vaccines.
- Children under the age of 6 months are not eligible to receive COVID-19.
How to prepare for your appointment:
- Everyone (including children) must present some form of identification. Bring your Ontario health card (preferred) or any of the following: driver’s license, birth certificate, Ontario photo card, passport, piece of registered mail, pay stub, student card, library card, club or organization ID cards, government-issued identification from jurisdictions outside of Ontario.
- It is recommended a child comes to the clinic with just one parent/legal guardian
- Some people may continue to wear masks, and others may not. Remember to be kind, understanding and respectful of people’s personal choices.
- Stay home if you have symptoms, or feeling unwell, even it is a mild cough or sore throat
- Check your confirmation and reminder emails for further instructions.
For an in-office appointment call your local health unit:
- New Liskeard office: 1-877-442-1212
- Kirkland Lake office: 1-877-442-1212
- Englehart office: 1-877-442-1212

Who is currently eligible?
Everyone aged six months or over at the time of their appointment is currently eligible to receive a COVID-19 vaccine.
Consistent with NACI, the Ontario Ministry of Health recommends a dose of the COVID-19 mRNA vaccine for individuals in the authorized age group (i.e. 6 months and older) who have been previously vaccinated against COVID-19, if it has been 6 months from the previousCOVID-19 vaccine dose or known COVID-19 infection (whichever is later).
Immunization is particularly important for those at increased risk of COVID-19. The Ontario Ministry of Health strongly recommends that individuals at high-risk from COVID-19, including those with a potential for greater impact from infection, receive a dose of the updated vaccine this fall, if it has been six months since their lastCOVID-19 vaccine dose or confirmed COVID-19 infection.
Staying Up to Date: Individuals 6 months and older are considered up to date with their COVID-19 vaccines if they have received a Fall 2023 COVID-19 dose.
What is the suggested timing between a previous COVID-19 infection and COVID-19 vaccination?
You should wait between 3 and 6 months to receive an updated COVID-19 vaccine after symptom onset or a positive COVID-19 test. Discussing the best timing for you with your healthcare provider is important. In certain circumstances, waiting six months may provide a better immune response.
For more information about COVID-19 vaccine administration guidance, click here.
What is an adverse event following immunization (AEFI) and how are AEFIs reported?
An adverse event following immunization (AEFI) is an unwanted or unexpected health effect that happens after someone receives a vaccine. AEFIs may or may not be caused by the vaccine. Monitoring AEFIs is an important step that helps make vaccination programs successful. All clients who receive the COVID-19 vaccine are told to contact their healthcare provider or THU if they experience an AEFI. When a healthcare provider reports an AEFI, they complete a form and then send it to their local public health unit for investigation and assessment.
All AEFIs are thoroughly investigated by public health nurses and signed off by the Medical Officer of Health. Most AEFIs are local reactions that resolve on their own.
Public Health Ontario releases a report about all AEFIs in the province. The report is updated weekly. You can read it here.
For more information about AEFIs and Ontario’s COVID-19 vaccine program, click here.

For more information about COVID-19 vaccination data in Ontario, click here.

Fertility, Pregnancy, and COVID-19 Vaccines
COVID-19 vaccines are safe if you are pregnant or planning to conceive
You can safely get the COVID-19 vaccine before becoming pregnant or in any trimester of pregnancy. It is also recommended that you stay up-to-date with booster doses. Please consult with your health care provider if you have questions about vaccination and pregnancy.
Getting the COVID-19 vaccine while you’re pregnant, breastfeeding, or trying to conceive is safe and highly recommended by:
Several studies have demonstrated that vaccination in pregnancy has no impact on:
- pregnancy outcomes (including miscarriage, premature birth, fetal growth restriction, and high blood pressure during pregnancy)
- medical complications of pregnancy
- maternal death
The benefits of getting vaccinated to prevent potential complications in pregnancy far outweigh the risks. The vaccine will protect you from COVID-19, and it will also reduce the risk of severe illness and complications related to COVID-19 in pregnancy. Studies suggest that after vaccination you will pass antibodies to your baby, which may keep them safe after birth.
For more information about COVID-19 vaccines in pregnancy, click here.

20250613/kp:tr